Abstract
Background: BCMA targeted CAR T cell therapy has shown promising results in patients with relapsed/refractory multiple myeloma (RRMM), but relapses are common. Additional treatment options with novel therapeutic targets or mechanisms of action are needed. Here we report on the safety and efficacy of MCARH109, the first-in-class G Protein Coupled Receptor Class C Group 5 Member D (GPRC5D) targeted CAR T cell therapy (Smith EL et al. Sci. Trans Med 2019) in RRMM including patients who relapsed post BCMA targeted CAR T cell therapy.
Methods: This is a phase I first-in-human, dose escalation trial of MCARH109; patients received lymphodepleting chemotherapy with fludarabine 30 mg/m 2 daily and cyclophosphamide 300 mg/m 2 daily for 3 days followed by a single infusion of MCARH109. The trial followed a standard 3+3 design with the following dose cohorts to date: 25X10 6, 50X10 6, 150X10 6, 450X10 6 viable CAR + T cells. The primary objective is to assess safety of MCARH109; secondary objectives include anti-myeloma efficacy, expansion and persistence of MCARH109 using quantitative polymerase chain reaction (qPCR) on peripheral blood and bone marrow samples.
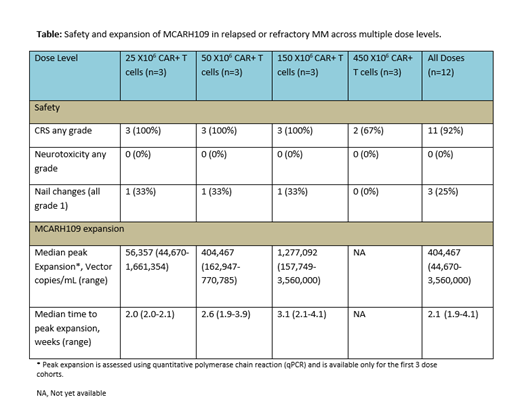
Results: 18 patients with RRMM were enrolled and underwent apheresis between September 15, 2020 and July 14, 2021. 12 patients have completed MCARH109 infusion to date, with 6 patients currently undergoing manufacturing and pending treatment. Of the 12 patients treated, median age was 59 (37-76) years and patients received a median of 8 (4-14) lines of therapy. 11 (92%) were penta-exposed, all patients were triple refractory, and 7 (58%) had prior treatment with BCMA targeted therapy including 6 (50%) who received prior BCMA CAR T therapy. 3 (25%) patients had non-secretory myeloma and 6(50%) patients had extramedullary plasmacytoma at baseline. 11 (92%) were refractory to last line of therapy and 11 (92%) patients received bridging therapy after apheresis prior to MCARH109 infusion; all patients were refractory to bridging therapy. There were no dose limiting toxicities. Cytokine release syndrome (CRS) grade 1-3 occurred in 11 (92%) patients with only one patient with grade 3 event; 4 (25%) patients received tocilizumab and 1 (8%) received dexamethasone for the treatment of CRS (Table). There were no neurologic toxicities reported to date; 3 (25%) patients had grade 1 nail changes possibly related to MCARH109 (Table). As of July 28, 2021, all treated patients have been followed for at least 2 weeks (median: 13.0 weeks; range: 2.0-39.1 weeks) and 10 (83%) had at least a minimal response or better (2 responses unconfirmed): 2 minimal response, 3 partial response, 3 very good partial response, 2 stringent complete response (sCR). 5 (56%) of the first 9 patients were minimal residual disease (MRD) negative in the bone marrow by multicolor flow cytometry (sensitivity: 10 -5). 6 (100%) patients with prior BCMA CAR T therapy had a response with 2 patients achieving sCR. We also noted robust MCARH109 expansion in the peripheral blood using qPCR across the first 3 dose levels with available data (peak expansion vector copy number/mL, median: 404,467; range: 44,670- 3,560,000; Table). With a median follow-up of 13 weeks, 9 (75%) patients are progression free and followed without additional therapy.
Conclusions: MCARH109 is the first-in-class GPRC5D targeted CAR T cell therapy for MM and has a very manageable safety profile with no serious or unexpected toxicities; this dose escalation study is ongoing with additional patients planned for treatment at higher doses. Efficacy is promising in heavily pre-treated RRMM, reflected in high rates of clinical response as well as MRD-negativity, including at doses as low as 25x10 6 CAR T cells. Clinically important, all 6 patients who relapsed after BCMA CAR T therapy responded to GPRC5D targeted CAR T therapy, including 2 patients who achieved sCR.
Mailankody: Allogene Therapeutics: Research Funding; Physician Education Resource: Honoraria; Bristol Myers Squibb/Juno: Research Funding; Takeda Oncology: Research Funding; Fate Therapeutics: Research Funding; Jansen Oncology: Research Funding; Evicore: Consultancy; Legend Biotech: Consultancy; Plexus Communications: Honoraria. Shah: Janssen: Research Funding; Celgene/BMS: Research Funding. Lesokhin: pfizer: Consultancy, Research Funding; Iteos: Consultancy; Trillium Therapeutics: Consultancy; Genetech: Research Funding; Serametrix, Inc: Patents & Royalties; bristol myers squibb: Research Funding; Janssen: Honoraria, Research Funding; Behringer Ingelheim: Honoraria. Korde: Medimmune: Membership on an entity's Board of Directors or advisory committees; Amgen: Research Funding. Hassoun: Celgene, Takeda, Janssen: Research Funding. Hultcrantz: GlaxoSmithKline: Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Research Funding; Daiichi Sankyo: Research Funding; Intellisphere LLC: Consultancy; Curio Science LLC: Consultancy. Shah: Amgen: Research Funding; Janssen: Research Funding. Landau: Takeda, Janssen, Caelum Biosciences, Celgene, Pfizer, Genzyme: Membership on an entity's Board of Directors or advisory committees; Takeda: Research Funding; Genzyme: Honoraria. Scordo: Angiocrine Bioscience: Consultancy, Research Funding; Omeros Corporation: Consultancy; Kite - A Gilead Company: Membership on an entity's Board of Directors or advisory committees; i3 Health: Other: Speaker; McKinsey & Company: Consultancy. Roshal: Auron Therapeutics: Other: Ownership / Equity interests; Provision of services; Celgene: Other: Provision of services; Physicians' Education Resource: Other: Provision of services. Landgren: Janssen: Other: IDMC; Janssen: Honoraria; Janssen: Research Funding; Amgen: Honoraria; Celgene: Research Funding; Amgen: Research Funding; Takeda: Other: IDMC; GSK: Honoraria. Dogan: Physicians' Education Resource: Honoraria; Seattle Genetics: Consultancy; Peer View: Honoraria; Takeda: Consultancy, Research Funding; Roche: Consultancy, Research Funding; EUSA Pharma: Consultancy. Giralt: Actinnum: Membership on an entity's Board of Directors or advisory committees; JENSENN: Membership on an entity's Board of Directors or advisory committees; GSK: Membership on an entity's Board of Directors or advisory committees; AMGEN: Membership on an entity's Board of Directors or advisory committees; CELGENE: Membership on an entity's Board of Directors or advisory committees; PFIZER: Membership on an entity's Board of Directors or advisory committees; JAZZ: Membership on an entity's Board of Directors or advisory committees; SANOFI: Membership on an entity's Board of Directors or advisory committees; BMS: Membership on an entity's Board of Directors or advisory committees. Park: Autolus: Consultancy; Kite Pharma: Consultancy; PrecisionBio: Consultancy; Minerva: Consultancy; Curocel: Consultancy; Intellia: Consultancy; Amgen: Consultancy; Affyimmune: Consultancy; Innate Pharma: Consultancy; Novartis: Consultancy; Servier: Consultancy; Kura Oncology: Consultancy; Artiva: Consultancy; BMS: Consultancy. Rivière: FloDesign Sonics: Other: Provision of Services; Juno Therapeutics: Patents & Royalties; The Georgia Tech Research Corporation (GTRC): Other: Provision of Services (uncompensated); Centre for Commercialization of Cancer Immunotherapy: Other: Provision of Services; Fate Therapeutics: Other: Provision of Services, Patents & Royalties. Brentjens: BMS: Consultancy, Patents & Royalties, Research Funding; Gracell Biotechnologies, Inc: Consultancy, Ended employment in the past 24 months; sanofi: Patents & Royalties; Caribou: Patents & Royalties. Smith: Eureka Therapeutics: Consultancy; Fate Therapeutics: Research Funding; Chimeric Therapeutics: Consultancy; Novarits: Consultancy; Sanofi: Patents & Royalties: GPRC5D antibody based therapies; BMS: Consultancy, Membership on an entity's Board of Directors or advisory committees, Patents & Royalties: CAR T cells for MM.
MCARH109 is an experimental GPRC5D targeted CART therapy

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal